The PERIGENOMED project

The PERIGENOMED project

Using genomic innovation for the screening of rare diseases in french newborns
Working together to build tomorrow's preventive noenatal medicine
The child's benefit at heart of our approach
A project focused on innovation in life sciences
Newborn screening (NBS) is a public health intervention designed to detect, from birth and before the onset of symptoms, certain rare and serious diseases, most of which are genetic. Diagnosis through NBS makes it possible to apply appropriate therapeutic or preventive measures to limit the impact of these diseases on newborn health. France was a forerunner when it set up its NBS program in 1972, but it now lags behind a number of European countries that currently screen for 20 or more rare diseases.
- Recent therapeutic innovations offer the opportunity to improve the health and quality of life of children with early-onset rare diseases.
- The development of next-generation genomic sequencing technologies over the last two decades, with the recent launch of new ultra-high-throughput sequencers. These new tools make it possible to screen for hundreds of rare diseases using a single biological test.
- Following these innovations, the 2021 revision of the bioethics laws approves the use of first-line genetic testing for NBS.
Given the ever-accelerating pace of therapeutic advances in rare diseases, adding new treatable diseases to the NBS on an individual basis will no longer be an efficient model in the future. It is therefore becoming essential to consider the use of new technologies that can easily increase the number of rare diseases screened, in line with these therapeutic advances. The new technologies for genome-wide high-throughput sequencing appear to be a highly suitable option, given their rapid progression.
A number of countries are currently considering the use of genome-wide analyses for NBS. These countries have formed a consortium of international scientific experts (ICoNS), which is working to integrate genomic innovation into NBS, by promoting the sharing of knowledge and experience.
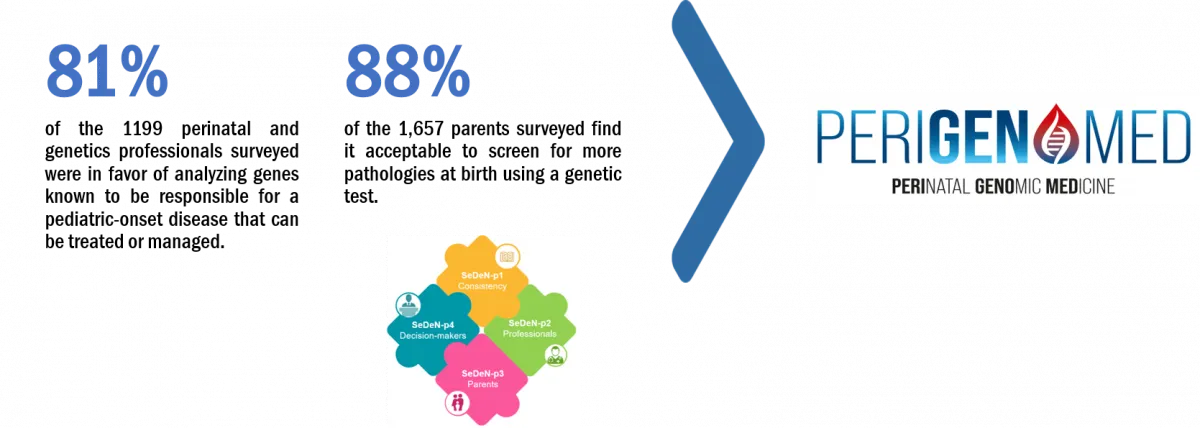
In 2013, the Fédération Hospitalo-Universitaire (FHU) TRANSLAD (link to Home - Translad) was established. It was the first hospital federation dedicated to genomic medicine in France. Translad was a national pioneer in setting up projects involving genomic innovation in healthcare, particularly in emergency contexts (intensive care and pregnancy). In 2020, in conjunction with the Société Française de Dépistage Néonatal (SFDN), it launched the SeDeN project, focusing on the social acceptability of extending NBS to include genomic sequencing technologies. The highly positive feedback from professionals and parents support the development of this project. It is thus essential to move towards the extension of NBS through genome-wide high-speed sequencing in France.
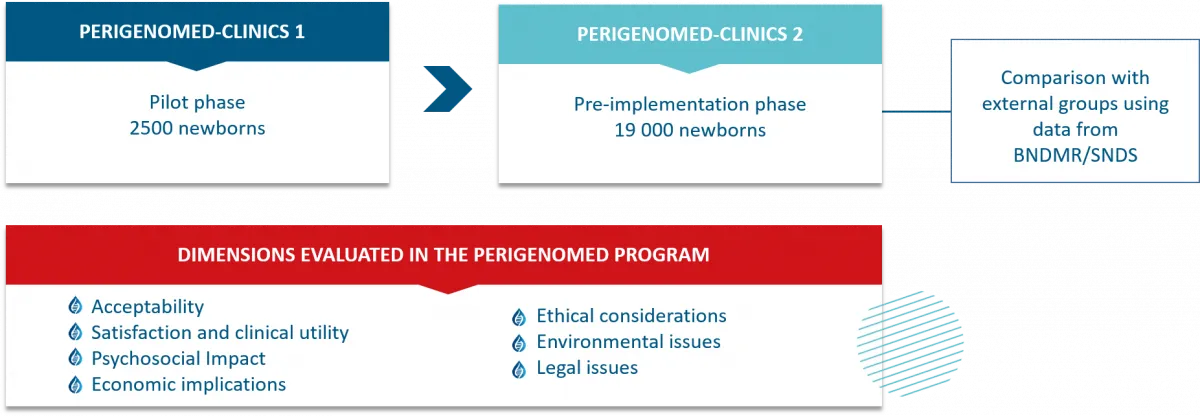
The PERIGENOMED project aims to assess the feasibility and effectiveness of expanding a neonatal screening program through genomic sequencing and to gather evidence supporting its deployment in France. It proposes to screen for more than 800 rare diseases for which early intervention provides substantial benefits for the child, including presymptomatic treatment, early tailored care, access to therapeutic innovations, improved clinical outcomes, and enhanced quality of life. PERIGENOMED incorporates an in-depth analysis of the organizational, regulatory, legal, ethical, psychosocial, economic, and environmental challenges associated with genomic neonatal screening (GNS).
The first phase, PERIGENOMED-CLINICS 1, conducted in collaboration with the partner University Hospital Federation (FHU) GenOMedS (FHU GENOMEDS Rare and Omics Diseases), is funded with approximately 3 million euros through a public-private-associative partnership. This phase will offer prospective parents of 2,500 newborns in five university hospitals (Dijon, Besançon, Rennes, Nantes, and Angers) screening for about 400 treatable diseases, with the option to screen for an additional 400 actionable diseases. Its objective is to test the implementation pathways for GNS and the subsequent care processes.
The second phase, PERIGENOMED-CLINICS 2, designed as a large-scale pilot program, aims to evaluate the deployment of GNS under real territorial conditions and assess its impacts. Scheduled to begin in 2026, it is expected to include approximately 19,000 newborns from maternity hospitals across five departments in the Bourgogne-Franche-Comté region. All included newborns will be monitored for five years. Those diagnosed with a confirmed rare genetic disease will receive specialized follow-up care at a designated rare disease reference or expert center. The estimated budget for this phase exceeds 10 million euros, and its implementation will require government funding.
The expected benefits of a DNNg could be many:

PERIGENOMED was designed with a participatory approach, involving:
- National rare disease health networks
- Genomic medicine stakeholders
- Neonatal screening experts and scientific societies
- Humanities and social sciences research teams
- Industrial partners
- Sponsors and patient associations


In line with the third national program for rare diseases (3ème Plan National Maladies Rares, PNMR3), the PFMG2025 (Genomic Medicine France 2025) and the upcoming PNMR4, PERIGENOMED is a means of anticipating the future. It will also serve as an accelerator for French industry around genomic medicine, and is fully in line with the aims outlined by the PFMG2025. PERIGENOMED is designed to help France meet its objective of becoming a pioneering force in the field of genomic medicine.